
Biotechnology
China has significantly bolstered its biotechnology sector, prioritizing it in three of the seven cutting-edge science and technology fields in its 14th Five-Year Plan (2021-2025): brain science, genetic engineering, and clinical medicine. Biotech is also crucial to global development goals, particularly in public health, sustainable energy, and food security.
With sizeable domestic support, Chinese companies such as BGI (genome research), Beigene (anti-cancer drugs), Mindray (medical equipment) and WuXi Apptec (services and equipment for drug development) have risen to global prominence.
ChemChina took over Syngenta, the world’s third largest seed producer, in 2017. China is the world’s largest exporter of Active Pharmaceutical Ingredients (APIs).
International collaboration and competition in biotech are intricately intertwined. China participates in roughly a third of the world’s multiregional clinical trials – only the US participates in more. China has also welcomed large investments by multinational pharmaceutical companies in domestic startups. At the same time, fair competition and market access remain a problem for many foreign firms in the healthcare sector, as localization efforts give domestic firms preferential treatment.
Great power competition adds further complexity. China, the US, and Europe all scrutinize the sharing of sensitive genetic and medical data. They also race for supremacy in dual-use technologies, such as brain-computer interfaces, which could be used for military robots.
Graphics dashboard
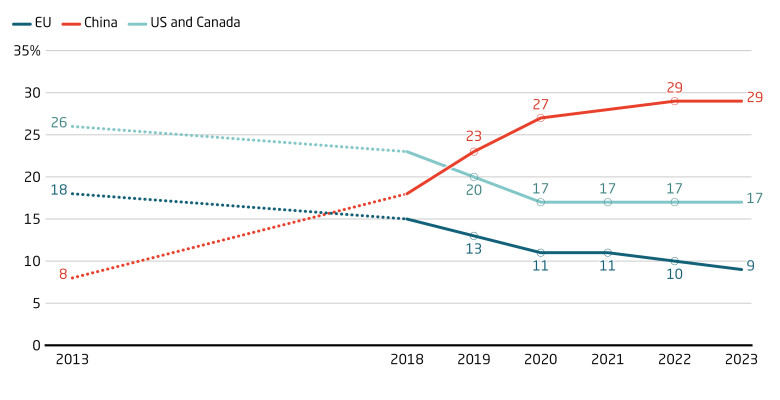
Between 2013 and 2023, China’s slice of global clinical trial starts jumped from 8 percent to 29 percent, overtaking Europe and closing in on North America. Beijing has successfully promoted China as a location for clinical trials. Although getting official approval in China often still takes longer than in Europe, its large public hospitals significantly reduce patient recruitment timelines and costs.
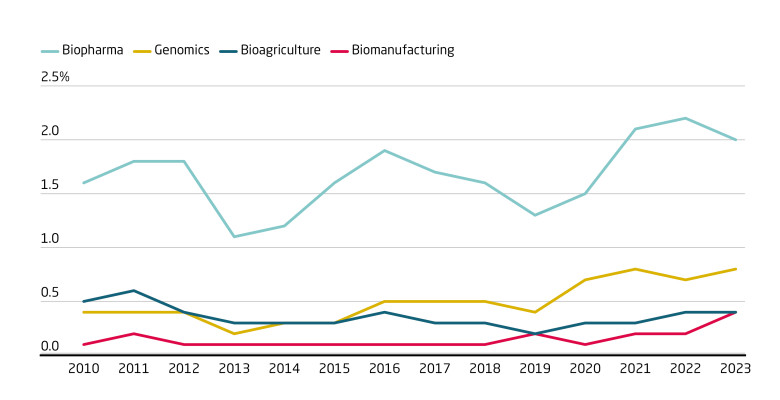
Tracking the share of national and provincial documents that mention key terms reveals that biopharma and genomics consistently command the most policy attention, while bio-agriculture and, in particular, biomanufacturing trail far behind. This policy bias correlated with the areas in which China has made most progress: drug discovery, medical contract research and genomic sequencing. Attention for bio-based industrial production is increasing only slowly, suggesting that a government -push (which will be required to increase adoption) is unlikely in the near future.
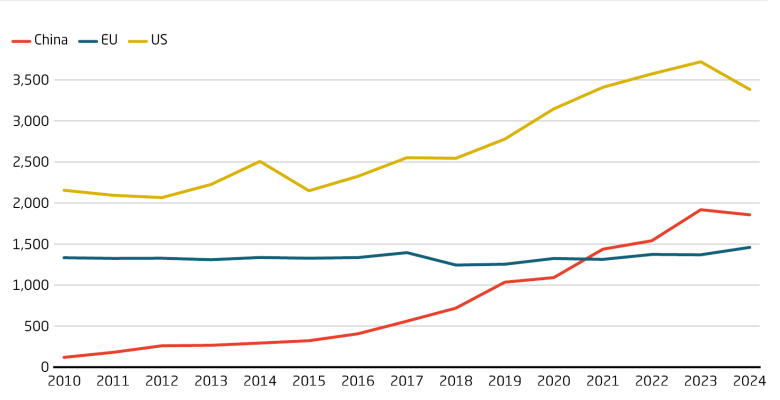
While the US is still the unchallenged leader, China has rapidly increased its number of invention patents over the last 20 years. It overtook the EU in 2021. China’s rise in global patent applications is consistent with increased interest from overseas investors in its biotech startups. In pharma, Chinese firms are getting increasingly successful in discovering me-too, me-better and more recently first-in-class drugs.
Biotechnology in China: Timeline of crucial events
China approves its first COVID-19 vaccine for general public use, developed by state-backed pharmaceutical giant Sinopharm and the Pfizer/BioNTech vaccine was approved for use in the EU.
Chinese medical device company Mindray Bio-Medical Electronics acquires Finnish biotech company HyTest Invest and its subsidiaries for EUR 532 million.
Leading Chinese medical imaging firm United Imaging invests EUR 410 million in a new manufacturing and research facility in Shanghai to rely heavily on automatic and intelligent processes.
The National Development and Reform Commission releases the 14th Five-Year Plan for the bioeconomy, covering life sciences and biotechnologies and calling for more investment in basic research.
MIIT calls for companies to participate in a new biomedical materials innovation program to increase collaborative innovation with a focus on polymer, metal and inorganic non-metallic materials.
MOST issues clarifying rules on human genetic resources, preventing foreign entities from collecting resources in China and restricting their access to them.
AstraZeneca acquired Gracell Biotechnologies for USD 1.2 billion, marking the first time a multinational drugmaker fully acquires a Chinese biotech firm.
The National Healthcare Security Administration aims to enhance coordination between local governments on centralized or volume-based procurement (high volume, low cost purchase of pharmaceuticals).


MIIT issues procurement guidelines, placing restrictions on a wide range of medical equipment, also X-ray machines, MRI & surgical equipment. The rules require 25, 50, 75 or 100 percent local content.
MIIT releases Five-Year Plans for the medical equipment & pharma industries, to ensure basic supplies & improve the industrial chain, enhance pharma innovation, modernization, & supply security 2025.
Chinese healthcare companies such as Andon Health and Beijing Hotgen Biotech see revenue surge thanks primarily to overseas sales of COVID-19 home testing kits.
Fosun Pharma and Genuine Biotech win the right to market the first China-made oral Covid-19 drug. The EU approved the first oral COVID-19 antiviral treatment, Pfizer’s drug Paxlovid, in January 2022.
MIIT releases a three-year plan to develop China’s non-food bio-based materials industry. By 2025, it aims for strong innovation capabilities to broaden its circular economy & reduce carbon emission.
Neusoft Medical launches China's first dual-energy 3.0T magnetic resonance imaging system. Chinese firms can make systems between 1.5T to 3.0T (magnetic field strengths) using only domestic inputs.
Caixin reports: China's outbound deals involving treatment rights exceeded inbound deals for the first time in 2023. Other parties can use a company’s products, technology or intellectual property.
Tech progress
- China’s ImmuneOnco Biopharmaceuticals has shown early promise in shrinking lung tumors. In a trial conducted in China, tumors diminished in 62 percent of patients with advanced non-small cell lung cancer (NSCLC) given IMM2510 in combination with chemotherapy. (Source (EN): fiercepharma, July 31, 2025)
Domestic dynamics
- The MIIT issued a timeline for the brain-computer interface (BCI) industry towards 2030 that prioritizes healthcare, industrial, and consumer applications. China saw notable achievements, successfully implanting first-in-human BCI devices, while the US remains the front-runner in that field with firms such as Neuralink. (Source (CN): gov.cn, July 23, 2025)
- The NMPA approved 43 innovative drugs in H1 2025, up 59 percent over 2024. Reports show that approved drugs include a first-of-its-kind molecule in blood cancer and drugs for other conditions such as metabolic disorders and immune diseases. (Source (EN): SCMP, July 2, 2025)
- The Ministry of Industry and Information Technology unveiled its plan to establish over 20 pilot-scale biomanufacturing development platforms by 2027. It aims to help transition scientific research achievements from laboratories to large-scale industrial production, serving 200 enterprises and including over 400 products such as food and additives, biopharmaceuticals, cosmetics, chemicals, energy, and enzyme preparations. (Source (CN): gov.cn, June 12, 2025)
- China will include HPV vaccines into its national immunization program later this year, said Shen Hongbin, the director of the National Disease Control and Prevention Administration. Chinese media link this long-awaited addition (the first since 2008) to a Chinese breakthrough that reduces China’s reliance on the US firm Merck (Source (CN): STCN, September 11, 2025)
Foreign involvement
- On September 3, Swiss pharma company Novartis signed a licensing deal with Shanghai-based Argo Biopharmaceutical worth up to USD 5.2 billion, covering experimental RNAi drugs for cardiovascular diseases. (Source (EN): Reuters, September 3, 2025)
- The "Chang'an Pioneer Overseas Elite Talent Recruitment Program" was launched and aims to attract worldwide talent in biomedicine, electronic information, and advanced manufacturing to accelerate Xi'an's development as a technological innovation center and enhance cooperation along the Belt and Road Initiative. (Source (CN): STDaily, August 16, 2025)
Publications



